
Listen here: https://idiotspodcasting.buzzsprout.com/1782416/episodes/14999534-79-a-precis-on-penicillin-binding-proteins

G+ cell wall is basically one big block of peptidoglycan with some other proteins anchored in.
G- cell wall in detail:
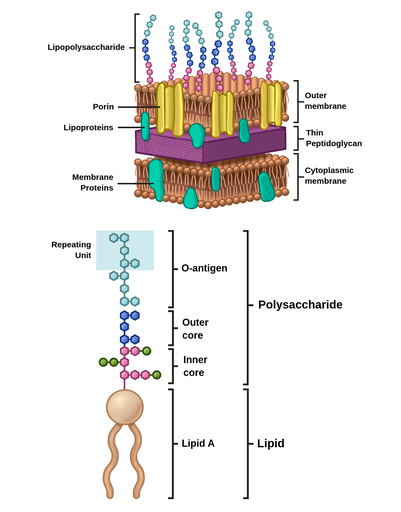
Periplasmic space = from surface of Cytoplasmic membrane to inside of Outer membrane, i.e. includes the peptidoglycan layer.
Outer membrane: Mostly composed of Lipopolysaccharide (LPS), the structure of which is shown below.
Also known as transpeptidation: This is what is catalysed by PBPs.
The Pentapeptide chain is composed of 5 amino acids: Alanine, Glutamine, Lysine, Alanine & Alanine

Note that D-glutamine and D-alanine are the only D-form amino acids found in nature (the rest are L-form), and are a feature of bacterial prokarya (i.e. they’re NOT found in Archaean prokarya or Eukaryotes)
Cross linking between G+ and G- cell wall: